
Rapamycin is the most promising aging intervention we currently have

It was in 1975 when scientists from Ayerst (now Pfizer) discovered a novel compound called rapamycin (also known as Sirolimus) in bacteria on Rapa Nui(Easter Island) in Chile. In 1999 rapamycin obtained FDA approval for the prevention of acute rejection of renal transplant. Unknown at the time, rapamycin would become the most potent anti-aging drug that humans currently hold.
This is the first article of a two-part series on rapamycin.
Rapamycin extends lifespan in all model organisms tested
The profound effect rapamycin has on lifespan was first observed in yeast cells, and later confirmed in every model organism tested, including the nematode C. elegans, fruit flies, and mice.
The results in mice were carried out by the Interventions Testing Program (ITP), the gold standard for lifespan experiments in mice.
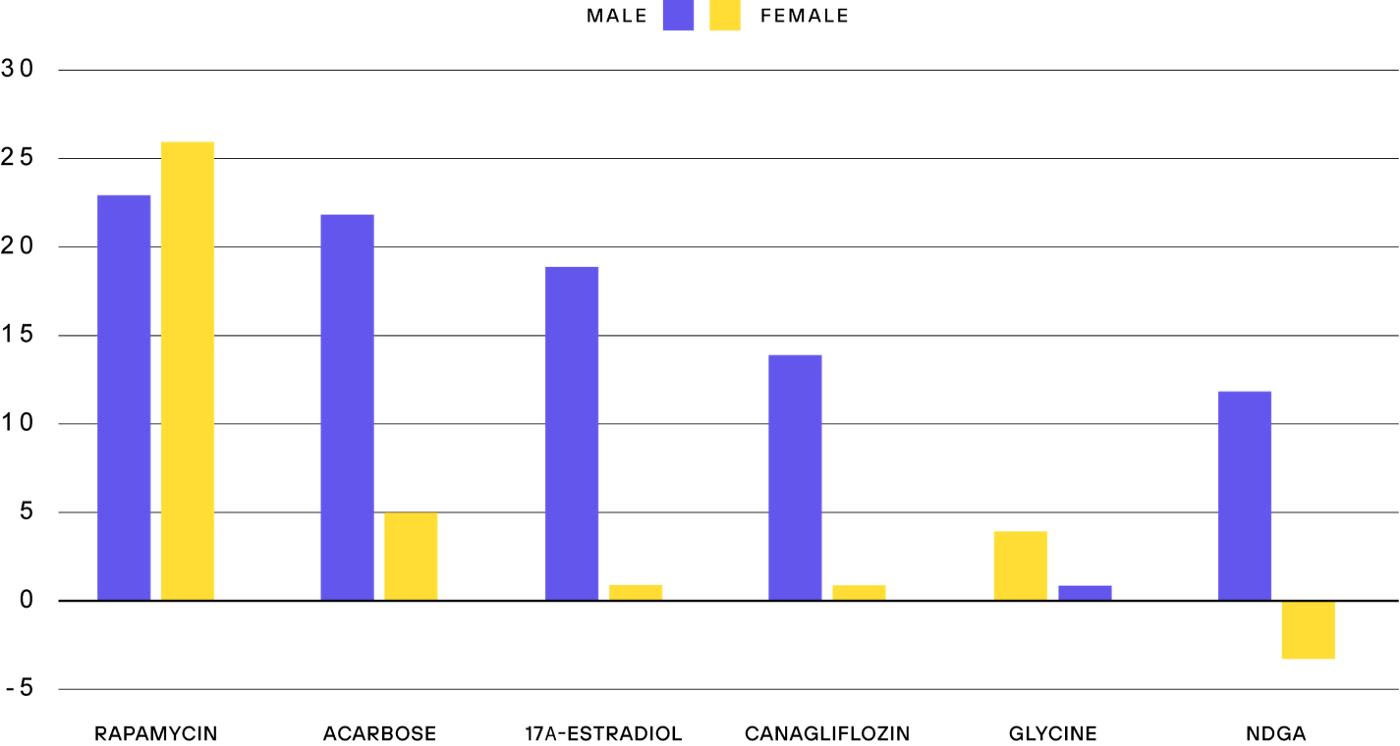
The first rapamycin experiment of the ITP was particularly interesting. Originally, the plan was to test the compound from young adulthood onwards. However, due to bioavailability problems, the mice were treated with rapamycin when they were already 600 days old, roughly corresponding to a 60-year-old human. Many thought that there was no way a drug can have an effect on aging when treatment starts at such a high age, but to everyone’s surprise, rapamycin still extended lifespan by up to 14%.
The effect of rapamycin on extending lifespan in mice has subsequently been replicated numerous times, and to this day rapamycin is the most effective drug at extending the lifespan of healthy mice, with increases of up to 26% when started earlier in life.
And since the dose and regimen for mice are not yet optimized, the maximum possible lifespan increase is probably even greater.
Another promising feature of rapamycin is that it works in both sexes, while many other interventions have beneficial effects either solely or predominantly in males, with little effect in female mice.
So, what is rapamycin doing to confer this longevity-promoting effect?
Rapamycin inhibits mTORC1 in a highly specific manner
Rapamycin works by inhibiting the function of an enzyme called mTOR (mechanistic target of rapamycin). mTOR is a master regulator of cell growth and metabolism.
mTOR is so critical to these essential processes that it can be found in all eukaryotic organisms. Furthermore, there is relatively little difference between mTOR in yeast and in humans, explaining the effectiveness of rapamycin at inhibiting mTOR in all animals tested. mTOR is an enzyme belonging to the class of kinases. Kinases work by adding phosphate groups to their substrates, which can cause conformational changes to the protein structure to regulate their function.
Rapamycin does not inhibit mTOR directly, but rather by forming a complex with a protein from the class of immunophilins called FKBP12. The resulting complex in turn binds mTOR and inhibits it allosterically. Allosteric inhibition means that the inhibitor slows down the enzyme, mTOR, without blocking its active site. The advantage of this mechanism is that rapamycin is highly specific to mTOR, compared to inhibitors that block the kinase function at the active site of mTOR directly, which tend to inhibit other kinases as well.
mTOR is part of two functionally distinct complexes, mTORC1, and mTORC2. Rapamycin only inhibits the mTORC1 complex directly, however, after chronic treatment with rapamycin mTORC2 levels decline as well. mTORC2 and mTORC1 functions are different. While the role of mTORC1 is well understood, many aspects of mTORC2 signaling remain elusive. We will focus on mTORC1 in this article, as it is the main target of rapamycin. We will cover the differences between mTORC1 and mTORC2 inhibition and its consequences in more detail in part two of this series.
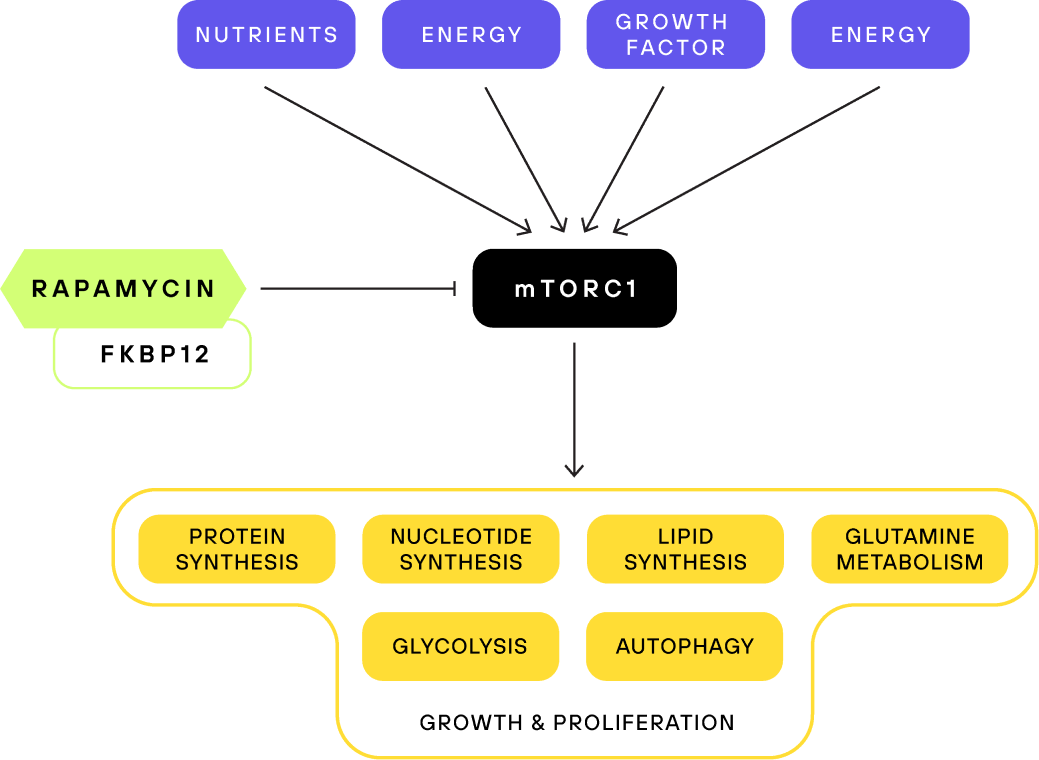
mTOR is a master regulator of nutrient sensing and growth signaling
The mTOR complexes are the main signaling nodes integrating environmental signals into cellular responses. If the conditions are favorable for anabolic processes, the mTOR pathway will initiate growth and proliferation.
To facilitate this, mTORC1 responds to nutrient availability, energy, oxygen status, and growth signals. If, for example, there is an abundance of amino acids, then mTORC1 will get activated and anabolic processes will get initiated.
As a result, active mTORC1 increases protein, lipid, and nucleotide synthesis, promotes mitochondrial biogenesis and energy production, and inhibits autophagy, among other processes. Basically, active mTORC1 is telling the cell to go full throttle. By inhibiting mTORC1, rapamycin tricks the body into reacting as though nutritional resources are scarce, thus activating protective pathways and mechanisms, like autophagy.
For that reason, rapamycin was initially seen as a mimetic of caloric restriction, one of the most potent lifespan-extending interventions. However, even though mTOR inhibition is crucial for the lifespan extension of caloric restriction, rapamycin and caloric restriction are still distinct in their effect.
Rapamycin positively influences all hallmarks of aging
One of the most influential papers in the field of aging is a 2013 review titled “The Hallmarks of Aging”. Here 9 hallmarks are attributed to aging, such as telomere attrition, epigenetic alterations, genomic instability, and others. Rapamycin influences all of them.
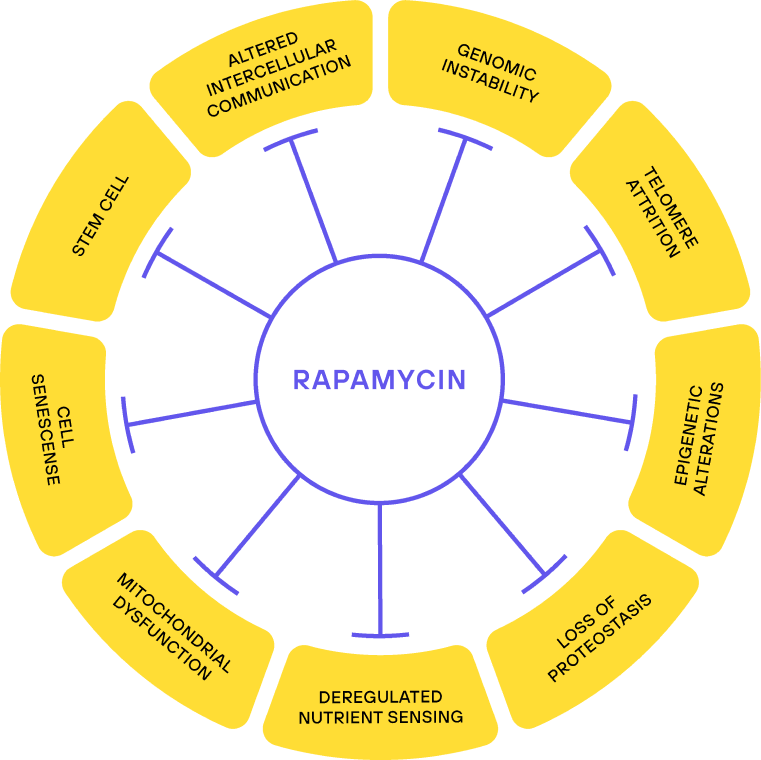
The hallmarks of aging do not necessarily reflect a causal explanation for aging. Still, they touch on most aspects of molecular changes seen with age and if a compound manages to influence all of them, then there is a good chance that itwill have an effect on healthspan/lifespan.
mTOR regulates mitochondrial processes, such as ATP production and translation of mitochondrial proteins. And indeed, rapamycin treatment has been shown to increase mitochondrial efficiency.
That rapamycin improves the hallmark of nutrient sensing is unsurprising, given that mTOR is the central nutrient sensor in the cell. Similarly, improved proteostasis is a logical conclusion due to the upregulation of autophagy by rapamycin.
Regarding epigenetic alterations, rapamycin has been shown in vitro to rejuvenate epigenetic markers of age. In animals, this connection is less clear. While rapamycin treatment reduces epigenetic age in mice, this was not observed in marmosets, a type of primate.
Rapamycin has also been characterized as a senomorphic, meaning it blocks the conversion of cells into senescent cells and reduces inflammatory phenotypes in senescent cells.
Beyond that, rapamycin rejuvenates hematopoietic stem cells, reduces DNA damage, and counteracts telomere attrition. It furthermore remodels the microbiome, which is proposed to be a novel hallmark of aging.
The improvements of many facets of aging upon rapamycin treatment thus support the idea that it targets aging itself. But why is mTOR inhibition seemingly more effective than many other interventions that also target age-related cellular changes?
Development might hold the answer to the anti-aging effects of rapamycin
An interesting observation is that the most potent anti-aging interventions are targeting pathways that are highly relevant to growth and development, like mTOR, Insulin-IGF, and AKT signaling. There seems to be a general trend that inhibiting pathways responsible for growth also slows aging. This suggests that the same pathways that promote growth actually cause aging in some way. We are joined by Professor Blagosklonny, one of the main proponents of this idea.
Interview with Prof. Mikhail Blagosklonny

AM: Why is it important to develop theories that try to explain the cause of aging?
MB: Well, it’s important because a correct theory can allude to the correct treatment of aging. Many theories of aging have been proposed over the years and a lot of them state that aging is solely caused by an accumulation of molecular damage, for example by free radicals. Therefore, antioxidants are widely used in the general population to slow down aging despite animal studies contradicting that free radicals cause aging and clinical trials showing no benefit. In 2006 I predicted based on the hyperfunction theory that rapamycin will extend the lifespan of animals, which was later confirmed.
So this is why it’s important: the theory can be translated into clinical practice.
AM: So does the accumulation of molecular damage play no role in aging?
MB: Molecular damage accumulates with age and after a long time it would kill the organism, but the organism generally dies from normal (mTOR-driven) aging before that happens. Molecular damage is not life-limiting. Accumulation of molecular damage could be artificially accelerated in animals. For example, by damage in enzymes that repair molecular damage. But in these cases, symptoms are different from symptoms of normal aging.
AM: If an accumulation of molecular damage is not the cause of aging, then what is, and how is it connected to mTOR (Target of Rapamycin)?
MB: The basis of the hyperfunction theory is that aging is a continuation of growth and development. Genes are highly regulated during development but fall into an evolutionary selection shadow post development and reproduction. Thus many genes show antagonistic pleiotropy.
Antagonistic pleiotropy describes the idea, that the same gene can have multiple effects (pleiotropic) that can be both beneficial and detrimental to the organism. Genes are selected for their beneficial traits at the beginning of life, even though they might be detrimental later in life. Evolutionarily, this detrimental effect later in life is not selected against as it only occurs after reproduction, thus lying in a selection shadow.
This means that the program responsible for growth and development, mainly the mTOR pathway, doesn’t get properly switched off after development, the pathway becomes hyperfunctional. This unwanted continuation becomes harmful and leads to the development of age-related diseases and aging. This explains why the genetic knockouts or interventions showing the greatest increase in lifespan are targeting growth-, and not damage repair signaling pathways. That hyperfunctional growth pathways drive age-related diseases is not just speculation. Involvement of mTOR was described in all human age-related diseases.
But while the hyperfunction theory is in complete agreement with evolutionary concepts like antagonistic pleiotropy, it is not an evolutionary but a mechanistic theory, since it can also give a mechanistic explanation of aging on the cellular level.
When cells cannot divide anymore they can enter a state of cellular senescence, which is characterized by hypertrophy (the cells become enlarged) and a hypersecretory phenotype. When I was working on cell growth and cellular senescence we found that cellular senescence is a continuation of cellular growth, when the cell cycle is arrested. Hyperfunctional growth signaling leads to a geroconversion from reversible cell cycle arrest into a senescent state. One of the main growth-signaling pathways is mTOR, and we showed that rapamycin can stop cells from becoming senescent. This cellular geroconversion is one of the mechanistic explanations for aging on an organismal level as well.
AM: How is mTOR regulated at different life stages and why does it become hyperfunctional?
MB: This is related, as mentioned, to antagonistic pleiotropy. Evolutionarily, it only matters what is beneficial early in life, for reproduction, so natural selection doesn’t regulate mTOR activity in later life. So, natural selection keeps mTOR activity optimal for fitness early in life, but too high for longevity late in life.
When the mTOR pathway is inhibited early in life, during development, it results in slow growth and decreased reproduction. These observations are made in laboratory animals, such animals will die in the wild of course, due to decreased fitness. But in laboratory conditions, they survive and live longer. Just recently it was published that inhibition of mTOR with rapamycin in young animals also extends lifespan.
But of course, this is not translatable to humans, we cannot treat infants or children with rapamycin. To slow their growth so they live longer, that would be completely absurd.
But fortunately, we don’t need to do this. You don’t need to inhibit development. But you can turn mTOR down pharmacologically in adults after development, when it becomes hyperfunctional, which I think holds great promise.
Disclaimer & Disclosure
DISCLAIMER: THIS ARTICLE DOES NOT PROVIDE MEDICAL ADVICE. No material in this article is intended to be a substitute for professional medical advice, diagnosis, or treatment. The text, images, and other material contained in this article are for informational purposes only.
VitaDAO has funded a clinical trial by Dr. Brad Stanfield on exercise and Rapamycin dosing. Pfizer has recently joined VitaDAO as a strategic contributor.


