Shining a Light on Optogenetics: An Innovative Approach to Combat Aging

Mitochondria in the spotlight: Powerhouses Dimming with Age?
In the intricate ballet of cellular activity, mitochondria often play a leading role. These double-membraned organelles are frequently referred to as the "powerhouses" of the cell, converting nutrients into energy that fuels nearly everything we do. But, as with all things, age takes its toll on them (Clemente-Suárez et al., 2023).
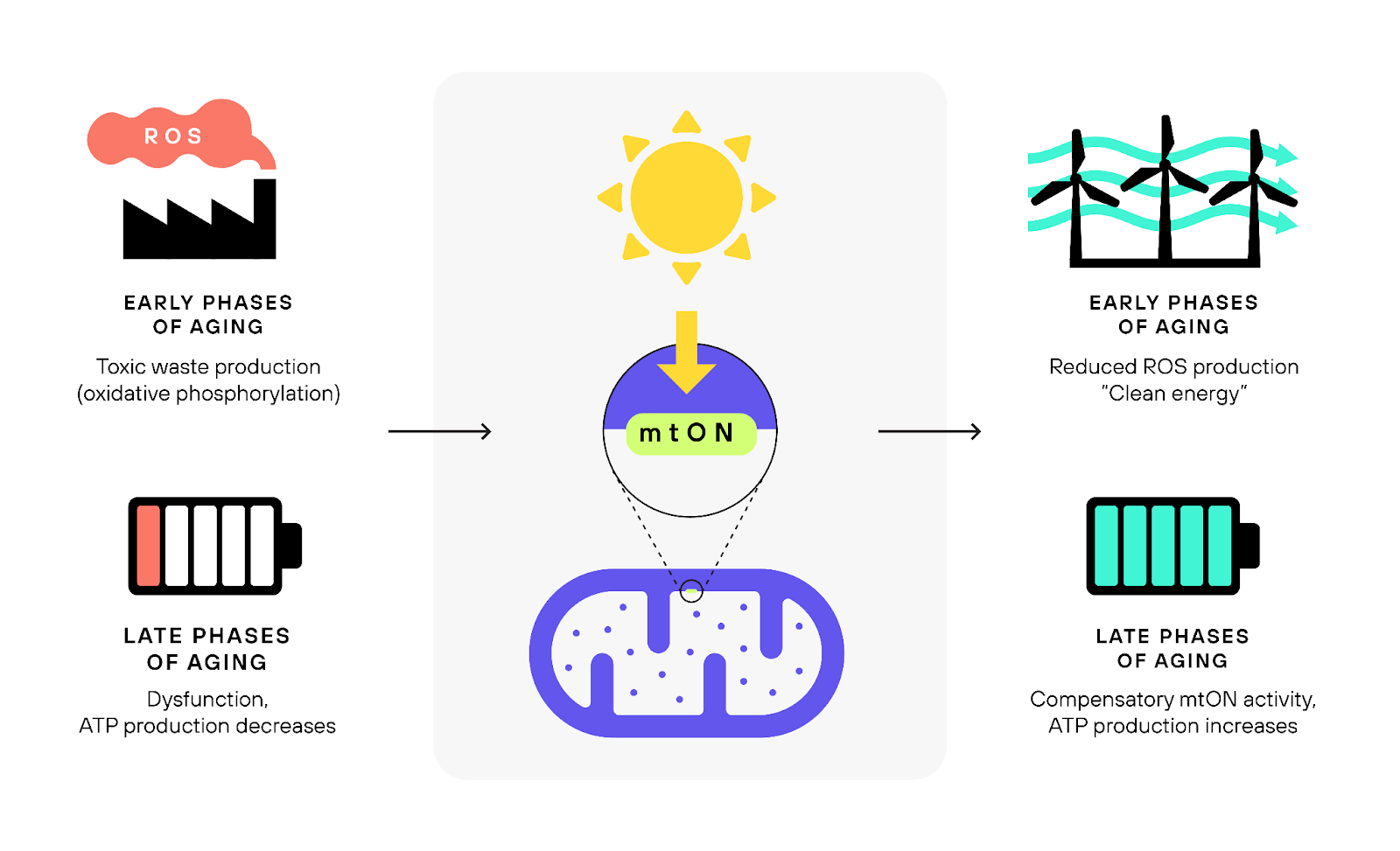
Mitochondrial dysfunction is one of the central features of aging (Payne & Chinnery, 2015). Many strategies designed to prolong life and maintain good health into old age focus on these cellular structures. From diets to drugs like metformin and rapamycin, various interventions aim to tweak mitochondrial metabolism (Amorim et al., 2022). Interestingly, many of these strategies work by reducing mitochondrial activity. This may sound counterintuitive, but certain studies have shown that decreasing specific aspects of mitochondrial processes can actually increase an organism's lifespan (B. Hwang et al., 2012; Campos et al., 2021). However, there's a catch. Reducing mitochondrial function often comes at the cost of the organism's vitality and its ability to handle stress (Aragoni Da Silva et al., 2023). Thus, the question remains: can such an approach be realistically applied to humans?
From Plants and Bacteria to Humans: A Radical Shift
All animals, including humans, need to consume other organisms for fuel, however some life forms, such as plants, algae and cyanobacteria have evolved to create their own fuel from sunlight, by mastering the art of solar power at the cellular level (Havurinne & Tyystjärvi, 2020).
It is thought that all organisms on the planet share a common ancestor, and thus many cellular mechanisms are universal, such as DNA for storing genetic information, ribosomes for producing proteins or the use of ATP as cellular energy currency. This allows opportunities for mechanisms from one organism to be utilised in another - take, for example, the CRISPR/Cas9 system, adapted from bacteria, that has revolutionized genetic editing in animals (Kick et al., 2017).
A Bright Idea: Optogenetics
To generate energy, our cells rely on a remarkable mechanism known as the electron transport chain. This process uses energy to push protons across the mitochondrial membrane, creating a kind of energy imbalance. Later, these protons can flow back through special channels, triggering the production of ATP. Think of it like how a battery operates. However, as we age, this process can become dysregulated, leading to a decrease in efficiency and even leakage of toxic free radicals.
The proposed strategy revolves around an engineered protein from fungal origin (Leptosphaeria maculans) called mtON, which is light-sensitive protein that pumps the aforementioned protons through the electron transport chain. When exposed to light, mtON pumps protons across the mitochondrial membrane, aiding in the creation of ATP, the cell's primary energy molecule (Tiwary et al., 2023). What's fascinating here is that this system allows a cell to produce energy from light, circumventing the regular process, and potentially rescuing dysfunctional mitochondria in aging cells (Berry & Wojtovich, 2020). This is not mere speculation. Groundbreaking experiments have already demonstrated that this light-based approach can enhance lifespan in worms (Berry et al., 2022).
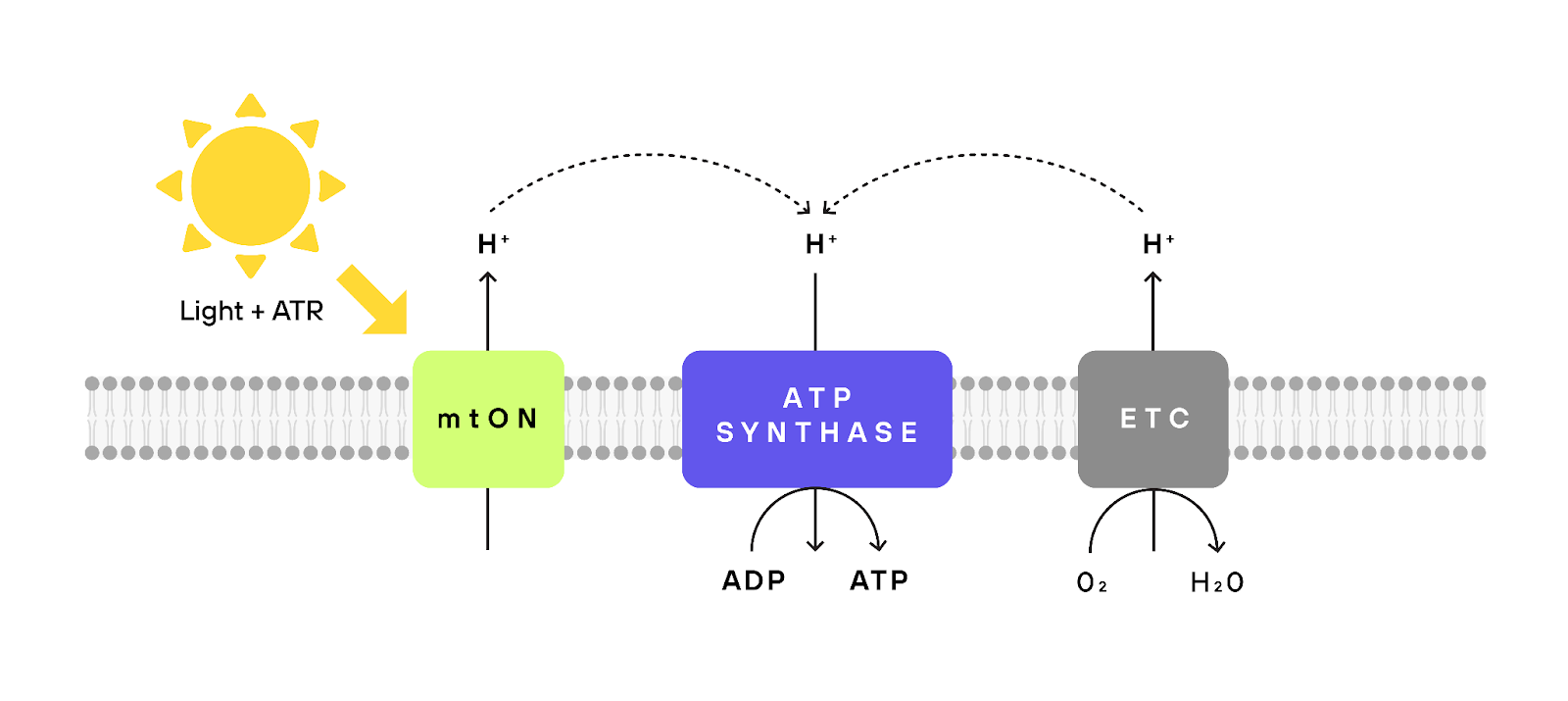
Harnessing the power of light for energy transduction in our cells could have profound effects on the way we age. By rejuvenating aging cells, the mtON approach offers a ray of hope for tissues that suffer from declining mitochondrial function. Aging cells, which struggle to produce adequate ATP due to faltering mitochondria (Berry & Kaeberlein, 2021), might find a new lease on life with the introduction of mtON.
Furthermore, aging isn't just about the passing of time; it's also about the wear and tear our cells experience. With ATP potentially being produced from light, we might see a decrease in overall cellular metabolic activity. This translates to reduced waste and a subsequent slowdown in the accumulation of cellular damage. Importantly, there might also be a drop in oxidative stress, a significant contributor to the aging process (Warraich et al., 2020).
One of the most bold potential implications revolves around our dietary needs. While this sounds like science fiction, maybe in the future, the light-driven energy transduction could even reduce our reliance on food for energy. This could allow us to enjoy the benefits associated with calorie restriction, which is known to promote health and longevity (Dorling et al., 2020), without the associated downsides of reduced energy intake. This shift might redefine our relationship with food and our understanding of metabolic health. But enough daydreaming for now…
The Future is Bright
To sum up, using light as an alternative energy source at the cellular level could pave the way for groundbreaking treatments against aging. While this hypothesis is still in its early stages, its potential is vast. Perhaps one day, instead of just basking in the sun for warmth, we might be doing it to recharge our cells!
Proposal author and Longevity Prize 3rd place winner: Dr Shahaf Peleg and Dr Andrew Wojtovi
Writer: Maria Marinova
Editors: Adrian Matysek, Rhys Anderson
References
Amorim, J. A., Coppotelli, G., Rolo, A. P., Palmeira, C. M., Ross, J. M., & Sinclair, D. A. (2022). Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nature Reviews Endocrinology, 18(4), 243–258. https://doi.org/10.1038/s41574-021-00626-7
Aragoni Da Silva, J., Rolland, Y., Martinez, L. O., & De Souto Barreto, P. (2023). Mitochondrial Dysfunction and Intrinsic Capacity: Insights From a Narrative Review. The Journals of Gerontology: Series A, 78(5), 735–742. https://doi.org/10.1093/gerona/glac227
B. Hwang, A., Jeong, D.-E., & Lee, S.-J. (2012). Mitochondria and Organismal Longevity. Current Genomics, 13(7), 519–532. https://doi.org/10.2174/138920212803251427
Berry, B. J., & Kaeberlein, M. (2021). An energetics perspective on geroscience: Mitochondrial protonmotive force and aging. GeroScience, 43(4), 1591–1604. https://doi.org/10.1007/s11357-021-00365-7
Berry, B. J., Vodičková, A., Müller-Eigner, A., Meng, C., Ludwig, C., Kaeberlein, M., Peleg, S., & Wojtovich, A. P. (2022). Optogenetic rejuvenation of mitochondrial membrane potential extends C. elegans lifespan. Nature Aging, 3(2), 157–161. https://doi.org/10.1038/s43587-022-00340-7
Berry, B. J., & Wojtovich, A. P. (2020). Mitochondrial light switches: Optogenetic approaches to control metabolism. The FEBS Journal, 287(21), 4544–4556. https://doi.org/10.1111/febs.15424
Campos, J. C., Wu, Z., Rudich, P. D., Soo, S. K., Mistry, M., Ferreira, J. C., Blackwell, T. K., & Van Raamsdonk, J. M. (2021). Mild mitochondrial impairment enhances innate immunity and longevity through ATFS‐1 and p38 signaling. EMBO Reports, 22(12), e52964. https://doi.org/10.15252/embr.202152964
Clemente-Suárez, V. J., Redondo-Flórez, L., Beltrán-Velasco, A. I., Ramos-Campo, D. J., Belinchón-deMiguel, P., Martinez-Guardado, I., Dalamitros, A. A., Yáñez-Sepúlveda, R., Martín-Rodríguez, A., & Tornero-Aguilera, J. F. (2023). Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines, 11(9), 2488. https://doi.org/10.3390/biomedicines11092488
Dorling, J. L., Martin, C. K., & Redman, L. M. (2020). Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Research Reviews, 64, 101038. https://doi.org/10.1016/j.arr.2020.101038
Havurinne, V., & Tyystjärvi, E. (2020). Photosynthetic sea slugs induce protective changes to the light reactions of the chloroplasts they steal from algae. eLife, 9, e57389. https://doi.org/10.7554/eLife.57389
Kick, L., Kirchner, M., & Schneider, S. (2017). CRISPR-Cas9: From a bacterial immune system to genome-edited human cells in clinical trials. Bioengineered, 8(3), 280–286. https://doi.org/10.1080/21655979.2017.1299834
Mansouri, M., Strittmatter, T., & Fussenegger, M. (2019). Light‐Controlled Mammalian Cells and Their Therapeutic Applications in Synthetic Biology. Advanced Science, 6(1), 1800952. https://doi.org/10.1002/advs.201800952
Payne, B. A. I., & Chinnery, P. F. (2015). Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1847(11), 1347–1353. https://doi.org/10.1016/j.bbabio.2015.05.022
Tiwary, V., Galow, A.-M., Wojtovich, A. P., & Peleg, S. (2023). Using light to drive energy transduction in metazoan aging. Trends in Biochemical Sciences, 48(11), 920–922. https://doi.org/10.1016/j.tibs.2023.08.010
Warraich, U.-A., Hussain, F., & Kayani, H. U. R. (2020). Aging—Oxidative stress, antioxidants and computational modeling. Heliyon, 6(5), e04107. https://doi.org/10.1016/j.heliyon.2020.e04107


