Imagine a bustling factory, working tirelessly day and night, crafting intricate items with utmost precision. This isn't a scene from a high-tech industrial park; it's happening right now inside your body. Our cells host these marvelous 'protein factories' known as ribosomes. Their job is to translate the genetic blueprints (the mRNAs or copies of our transcribed genes from the cell's nucleus) into protein masterpieces vital for all living organisms on the planet. This is an essential part of life known as the central dogma of molecular biology.
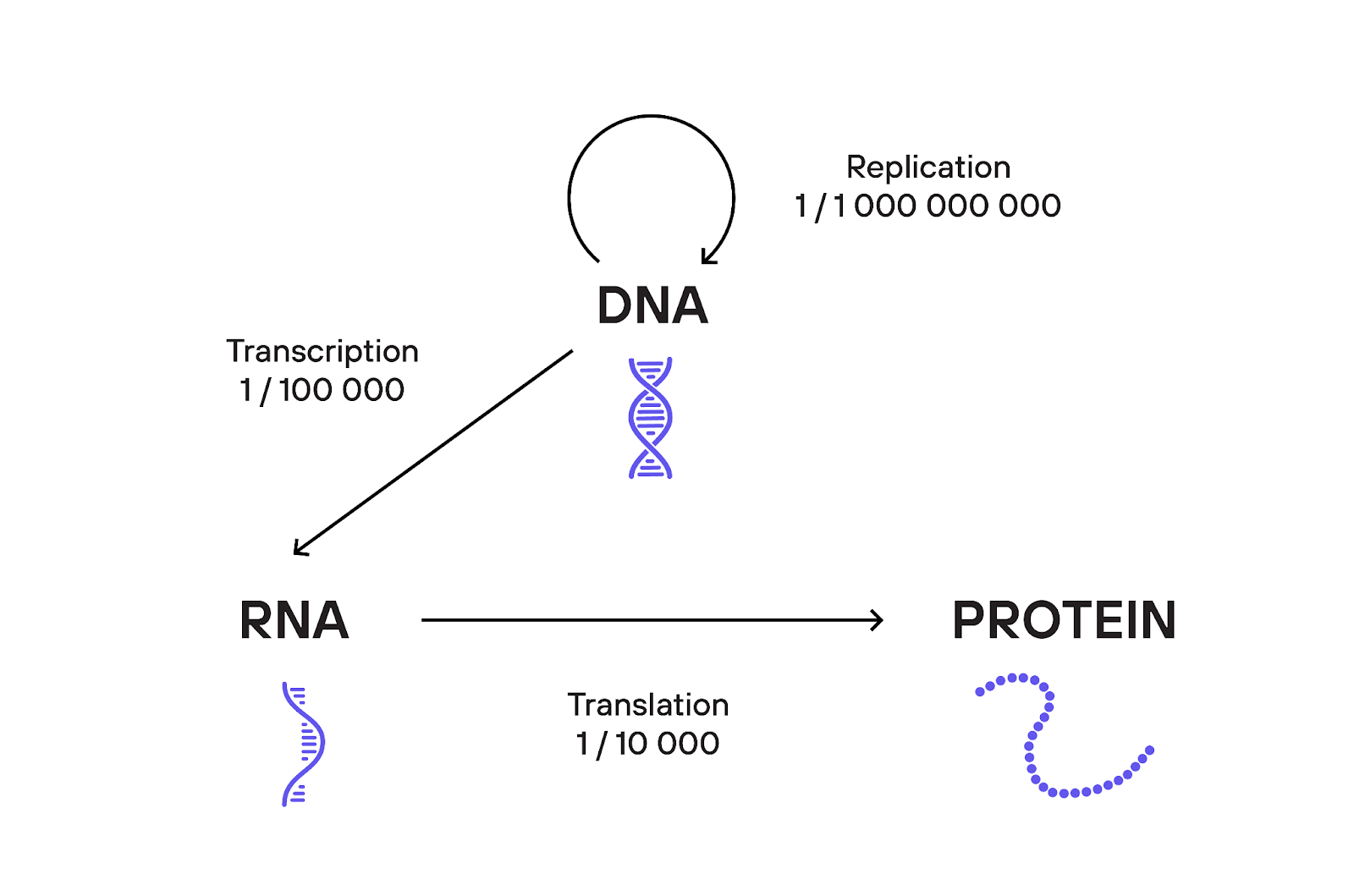
Typical error rates for each process of the “Central Dogma”
The ribosome is a cellular marvel, acting as the primary site of biological protein synthesis or translation. Like a seasoned craftsman reading a set of instructions to craft a product, ribosomes read mRNA sequences to stitch together the right sequence of amino acids and form proteins. However, as with any machinery, time can introduce wear and tear. It’s akin to the minute deviations that occur in a factory assembly line, where a widget produced on day one might not be identical to one made on day ten thousand. In the context of ribosomes and the biological setting, these inconsistencies, or 'noise', manifest as errors in protein synthesis. The process might result in proteins with incorrect amino acid sequences and misfolded structures.
Why does this matter?
Proteins, especially enzymes, rely on their exact structures to function properly. A slight deviation in structure might render a protein non-functional and harmful. This accumulation of 'noise' is a phenomenon that has been associated with the aging process itself and a plethora of age-related diseases (Anisimova et al., 2018).
In addition to the amino acid sequence, the structure, and function, of proteins can also be determined by post-translational modifications (PTMs), which are enzymatically regulated covalent conjugations of other molecules to specific amino acid residues on proteins. Perhaps the most widely studied PTM is phosphorylation, whereby the addition of a phosphate group creates a negative charge that can alter protein conformation, often activating the protein. Glycosylation is another PTM that involves the enzymatic addition of a sugar molecule, or glycan, to a specific residue - important in a wide variety of physiological functions from correct protein folding to stability. But there is another side of the coin, whereby proteins are subjected to non-enzymatic covalent additions of reactive metabolites. These are unregulated additions occurring at non-specific residues on the protein, which can negatively affect protein functionality and have mechanistic involvement in a range of pathologies, including aging (Harmel & Fiedler, 2018). Non-enzymatic glycosylation (addition of sugar molecules or glycation) is one such modification, which has implications in the progression of age-related pathologies and has been underappreciated for decades. The recent body of published research, linking glycation to the hallmarks of aging paradigm, has put it in a new light (Fedintsev & Moskalev, 2020).
Now, as we dive deeper into this intricate molecular dance, we encounter a significant player. Enter methylglyoxal (MGO), a byproduct of sugar metabolism (Allaman et al., 2015). MGO tends to get a bit too 'reactive' with our proteins and especially with certain amino acid residues, resulting in modifications that form various chemical adducts known as advanced glycation endproducts (AGEs) (Twarda-Clapa et al., 2022). These AGEs aren't benign; they've been linked with aging and an array of health conditions, such as diabetes and its complications (Singh et al., 2014).
A novel scientific inquiry is proposing an exciting conjecture linking back to MGO.
MGO, while modifying proteins, may also be throwing a ‘spanner’ in the works of our protein factories. This interference could increase the error rate of protein synthesis, potentially contributing to the disruption of protein homeostasis — the crucial equilibrium of protein production, function, and degradation — and driving us toward aging.
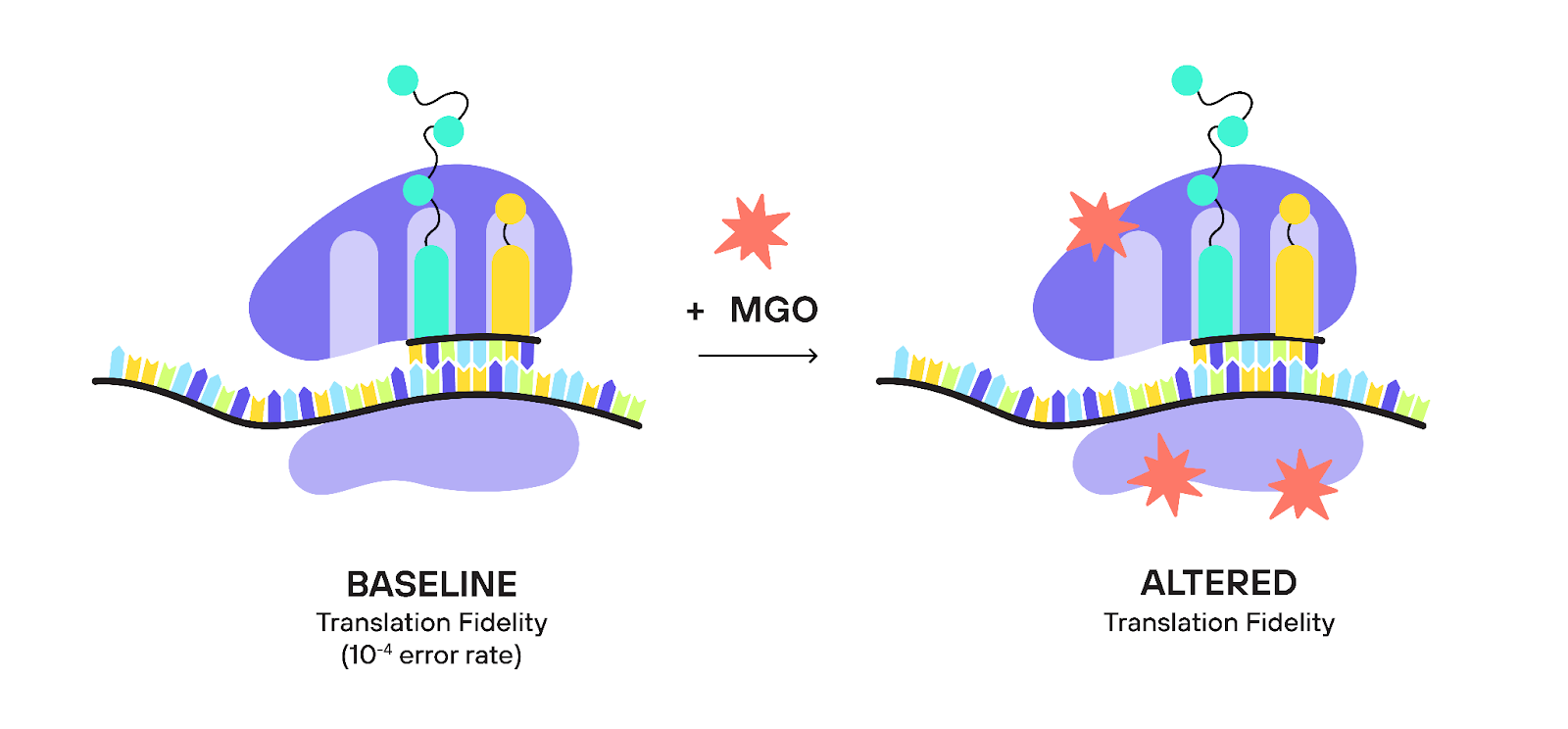
Why is this significant?
Well, longevity is, in part, a game of accuracy. The better our cells can convert genetic information into protein structures, the longer and healthier lives we're likely to enjoy. In fact, research has shown that some organisms with higher translation accuracy inherently have longer lifespans (Azpurua et al., 2013; Ke et al., 2017; Martinez-Miguel et al., 2021), while the artificial elevation of the translation error rate in animal models decreases lifespan (Brilkova et al., 2022; Moore et al., 2021; Shcherbakov et al., 2022).
By exploring how MGO, a common byproduct of our metabolism, could be meddling with this delicate process, researchers are laying the groundwork for potential interventions that could counter these effects and perhaps help us enjoy longer, healthier lives.
But the investigation doesn't stop at protein synthesis. The researchers are also probing how this process might interact with the environment of our cells, specifically the extracellular matrix (ECM). The ECM is a complex network of proteins that provide structural support to tissues and guide the behavior of cells (Theocharis et al., 2016).
What’s the working hypothesis here? Accumulation of AGEs might increase ECM stiffness, influencing cellular behavior and disrupting glucose uptake (Ge et al., 2021). This disruption could then lead to an uptick in MGO production and a subsequent rise in protein misfolding. It's an intricate cellular tug-of-war: stiffened ECM leads to increased MGO production and ribosome errors, which then contribute to aging and age-related diseases.
Pioneering experiments are already providing some answers. Researchers have shown that increasing MGO levels within cells, by stimulating the accumulation of its precursor, dihydroxyacetone phosphate, leads to more protein glycation. Also, a luciferase assay-based system — a clever new tool for measuring translation accuracy by detecting emitted light — is providing promising initial results.
With this line of inquiry and focusing on translational fidelity, we're getting closer to unravelling another aspect of the complicated biology of aging. In doing so, it's opening up potential routes for interventions that could reinforce our cellular accuracy, providing hope for healthier, more extended lives.
We're still in the early stages, but every discovery brings us closer to a future where aging is not an unavoidable decline but a process we understand and, crucially, can influence. Keep your eyes on this space; these seemingly innocent sugar byproducts could hold a piece to the intricate puzzle of longevity.
Authors: Rakhan Aimbetov & Maria Marinova
Editor: Rhys Anderson
Illustrator: Meghan Ho-Tong
References:
Allaman, I., Bélanger, M., & Magistretti, P. J. (2015). Methylglyoxal, the dark side of glycolysis. Frontiers in Neuroscience, 9, 23. https://doi.org/10.3389/fnins.2015.00023
Anisimova, A. S., Alexandrov, A. I., Makarova, N. E., Gladyshev, V. N., & Dmitriev, S. E. (2018). Protein synthesis and quality control in aging. Aging, 10(12), 4269–4288. https://doi.org/10/gfwdds
Azpurua, J., Ke, Z., Chen, I. X., Zhang, Q., Ermolenko, D. N., Zhang, Z. D., Gorbunova, V., & Seluanov, A. (2013). Naked mole-rat has increased translational fidelity compared with the mouse, as well as a unique 28S ribosomal RNA cleavage. Proceedings of the National Academy of Sciences of the United States of America, 110(43), 17350–17355. https://doi.org/10.1073/pnas.1313473110
Brilkova, M., Nigri, M., Kumar, H. S., Moore, J., Mantovani, M., Keller, C., Grimm, A., Eckert, A., Shcherbakov, D., Akbergenov, R., Seebeck, P., Krämer, S. D., Wolfer, D. P., Gent, T. C., & Böttger, E. C. (2022). Error-prone protein synthesis recapitulates early symptoms of Alzheimer disease in aging mice. Cell Reports, 40(13), 111433. https://doi.org/10/gq24jr
Fedintsev, A., & Moskalev, A. (2020). Stochastic non-enzymatic modification of long-lived macromolecules: A missing hallmark of aging. Ageing Research Reviews, 62, 101097. https://doi.org/10.1016/j.arr.2020.101097
Ge, H., Tian, M., Pei, Q., Tan, F., & Pei, H. (2021). Extracellular matrix stiffness: New areas affecting cell metabolism. Frontiers in Oncology, 11, 631991. https://doi.org/10.3389/fonc.2021.631991
Harmel, R., & Fiedler, D. (2018). Features and regulation of non-enzymatic post-translational modifications. Nature Chemical Biology, 14(3), 244–252. https://doi.org/10.1038/nchembio.2575
Ke, Z., Mallik, P., Johnson, A. B., Luna, F., Nevo, E., Zhang, Z. D., Gladyshev, V. N., Seluanov, A., & Gorbunova, V. (2017). Translation fidelity coevolves with longevity. Aging Cell, 16(5), 988–993. https://doi.org/10/gn9sgj
Martinez-Miguel, V. E., Lujan, C., Espie-Caullet, T., Martinez-Martinez, D., Moore, S., Backes, C., Gonzalez, S., Galimov, E. R., Brown, A. E. X., Halic, M., Tomita, K., Rallis, C., von der Haar, T., Cabreiro, F., & Bjedov, I. (2021). Increased fidelity of protein synthesis extends lifespan. Cell Metabolism, 33(11), 2288-2300.e12. https://doi.org/10/gnjjkx
Moore, J., Akbergenov, R., Nigri, M., Isnard-Petit, P., Grimm, A., Seebeck, P., Restelli, L., Frank, S., Eckert, A., Thiam, K., Wolfer, D. P., Shcherbakov, D., & Böttger, E. C. (2021). Random errors in protein synthesis activate an age-dependent program of muscle atrophy in mice. Communications Biology, 4(1), 703. https://doi.org/10/gkgp3t
Shcherbakov, D., Nigri, M., Akbergenov, R., Brilkova, M., Mantovani, M., Petit, P. I., Grimm, A., Karol, A. A., Teo, Y., Sanchón, A. C., Kumar, Y., Eckert, A., Thiam, K., Seebeck, P., Wolfer, D. P., & Böttger, E. C. (2022). Premature aging in mice with error-prone protein synthesis. Science Advances, 8(9), eabl9051. https://doi.org/10/gptgms
Singh, V. P., Bali, A., Singh, N., & Jaggi, A. S. (2014). Advanced glycation end products and diabetic complications. Korean Journal of Physiology & Pharmacology, 18(1), 1–14. https://doi.org/10.4196/kjpp.2014.18.1.1
Theocharis, A. D., Skandalis, S. S., Gialeli, C., & Karamanos, N. K. (2016). Extracellular matrix structure. Advanced Drug Delivery Reviews, 97, 4–27. https://doi.org/10.1016/j.addr.2015.11.001
Twarda-Clapa, A., Olczak, A., Białkowska, A. M., & Koziołkiewicz, M. (2022). Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells, 11(8), 1312. https://doi.org/10.3390/cells11081312