The Key to Longevity Could Be Hidden in Embryos

Scientists are forever searching for ways to rejuvenate aged cells and organisms back to a more youthful and healthier state — from stem cell therapies to telomere lengthening, or even the Frankenstein-esque heterochronic parabiosis experiments whereby older organisms receive the blood of younger ones. In 2012, the Nobel Prize in Physiology or Medicine was awarded to Sir John B. Gurdon and Shinya Yamanaka who discovered a technique to reprogram mature cells back to stem cells, coined induced pluripotent stem cells. Big pharma has attempted to use this knowledge to develop rejuvenating therapies, for example Altos labs became one of the highest funded start-ups in history with an initial investment of 3 billion dollars.
What if I told you the key to reverse aging might be hidden inside your very own cells? We’re not talking about sipping on an exotic “superfood” concoction, strapping on some high-tech gadget, or hiring a blood boy. The spotlight is firmly on embryogenesis — a biological process that we’ve all experienced yet barely understand.
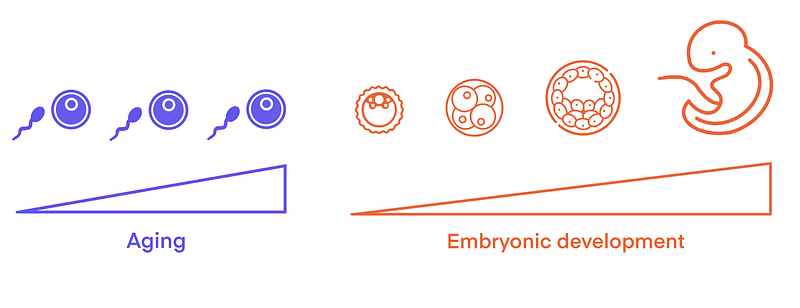
Okay, so what’s the big deal about embryogenesis? The miraculous transformation of two adult gametes (sex cells) into a full-blown organism effectively hits a giant reset button. Any age-related wear and tear present in the parent cells? Gone. Erased. Just like that, the biological clock is set back to zero. If that’s not a natural “Fountain of Youth,” what on earth is?
Like other adult cells, gametes undergo multiple types of age-related changes. They suffer mitochondrial dysfunction; they accumulate molecular damage and show epigenetic alterations [1, 2]. These afflictions bear the potential to be the unwelcome legacy passed down to the nascent embryo. Yet, this sparks a compelling hypothesis: could the embryo come equipped with inherent mechanisms to mitigate age related changes and undergo rejuvenation?
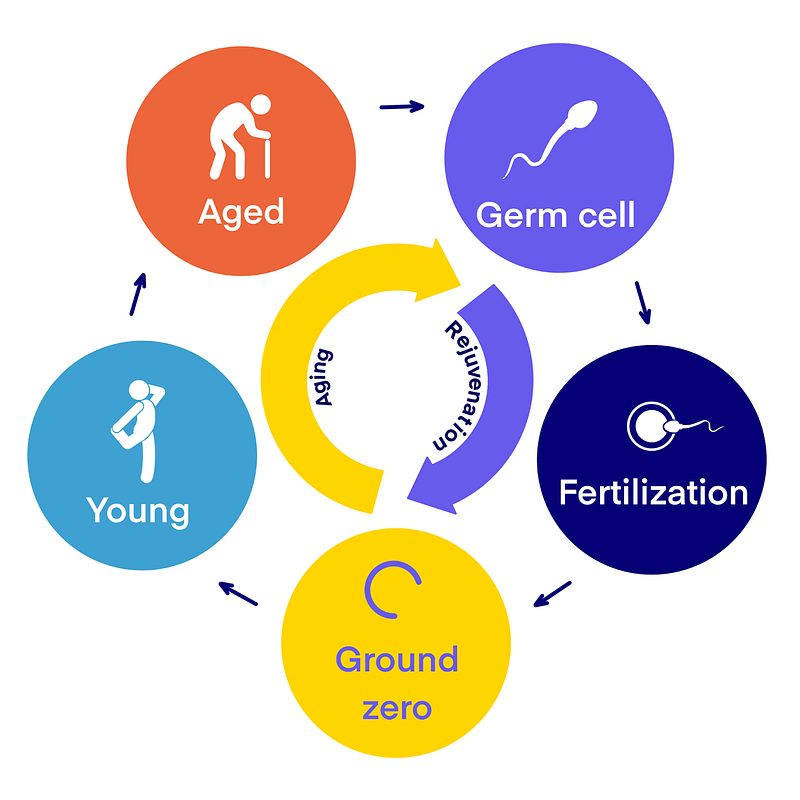
The idea of ‘rejuvenation’ isn’t new in the biological world. Conversion of somatic cells to induced pluripotent stem cells and the reset of the germline age with each generation are examples of this phenomena. This gives rise to the proposition of a ‘ground zero’ model, a mid-embryonic state characterized by the lowest biological age. It is hypothesized that the period from zygote to this ‘ground zero’ is associated with rejuvenation, wherein the biological age is decreased, telomeres are extended, and molecular damage is cleared. This concept of ‘ground zero’ helps illuminate the relationship between aging, development, rejuvenation, and de-differentiation, elements that are distinct throughout life [1, 3]. But here’s the million-dollar question: how does this occur? Well, hold onto your lab goggles, because scientists are already exploring this exciting frontier!
Using algorithms that can predict the age of an organism called epigenetic clocks, researchers have identified a point in embryogenesis where epigenetic age is reversed [1, 4]. Epigenetic clocks measure epigenetic age by looking at specific methylation patterns that are indicative of the age of an organism. Methylation patterns are a fundamental aspect of epigenetics. They involve the addition of a methyl group (a carbon atom linked to three hydrogen atoms) to the DNA molecule, typically at a cytosine nucleotide (one of the “building blocks” of DNA). These patterns play a pivotal role in gene expression because they can silence or activate genes without changing the underlying DNA sequence.
Scientists speculate that harnessing this natural epigenetic rejuvenation could be a key to promoting longevity and reducing age-related disease. This theory has taken the field by storm over the last few years and attracted a lot of commercial interest and new ventures trying to induce a state of “youthfulness” in cells by applying a process of epigenetic reprogramming.
Indeed, methylation presents a fascinating instance of the molecular alterations associated with aging. However, it represents only one facet of this complex process. A myriad of other molecular transformations and deteriorations in cellular function accumulate over time. The extent to which these changes affect gametes and whether they can be reversed by the embryo remains largely unknown.
To gain a deeper understanding of the relationship between aging and embryogenesis, researchers are delving deep, right into the core of gametes and embryos. Their goal? To identify any age-related alterations in parent cells that are subsequently erased as oocytes and sperm transform into an embryo, ultimately giving rise to a new organism.
The idea is to identify what molecular and functional changes occur as gametes age and to study whether embryogenesis has the capacity to reverse and alleviate some of them. To do this, scientists plan to use advanced technologies that can measure the abundance and state of a cell’s molecular components. These technologies include powerful tools like mass spectrometry which can be used to quantify and characterize proteins and the modifications they have undergone. This is important since protein damage and protein aggregates are thought to be a direct contributor to the aging process. Other tools like nanopore and SMRT sequencing have the potential to spot alterations in our DNA [5, 6]. These changes include various forms of DNA damage and understanding how these changes evolve with age and their behavior during embryogenesis, may uncover unknown aging and rejuvenation mechanisms.
However, the exploration doesn’t stop at proteins and DNA. Scientists are planning to do a deep dive into other components of cells, like lipids, metabolites, RNA, and the shape of DNA. They will gather heaps of data from these tests, then add functional data about different parts of the cells like mitochondria or lysosomes. When all these investigations come together, they would give us a detailed picture of how gametes age and how embryos deal with this.
These large datasets do more than just offer information about which facets of aging are undergoing rejuvenation. They also provide invaluable insights about the genes, peptides, and metabolites that drive the powerful genetic programs the embryo utilizes. This could prove very valuable, given that the embryo utilizes multiple processes that can be used to fight age related diseases.
For instance, in an intriguing series of experiments, scientists have injected cancerous cells into embryos. Yet, contrary to expectations, these malignant cells neither overwhelm the embryo nor are they eliminated. Instead, the embryo exercises a remarkable level of control over these intruders, compelling them to halt their proliferation [7]. Adding to the element of surprise is the fact that these transformed cells are not only maintained in tissues until birth, but they also undergo a complete reprogramming from their original cancerous state. It’s as if the embryo, in its potent nascent life, possesses a transformative capacity that can strip away the cells’ malignancy.
This intriguing process is likely to contribute to the intricate equilibrium required during tissue formation in embryogenesis. Unlike in cell culture where most cell types face a limit on the number of divisions, during embryogenesis the initial cells can form an entire organism without succumbing to replicative senescence. This is the result of elaborate cellular programs that grant cells the freedom to proliferate robustly while concurrently shielding them from becoming cancerous.
These are potent programs encoded within our very genome, holding potential applications for regenerating new tissue or combating malignancies in aging organisms. Indeed, certain creatures in the animal kingdom, like the aquatic hydra and some species of planaria, exploit similar processes to earn their ticket to immortality [8, 9]. Venturing into this new frontier of knowledge could unlock transformative therapeutic strategies, potentially reshaping our approach to age-related diseases and longevity.
While this all might sound like it’s straight out of a science fiction novel, it’s rooted in solid science. Even more exciting is that it’s mostly an uncharted territory! Despite the enormous potential of studying embryogenesis, it’s been an overlooked area in the longevity research world, making it ripe for exploration. Cracking the code of embryogenesis could very well be the key to understanding aging and how to reverse it. If successful, this could be a game-changer in how we approach aging and longevity. Forget about mythical fountains or enchanted forests; our ticket to timeless youth might just be nestled within the miracle of life itself!
Authors: Carlos Galicia & Maria Marinova
Editors: Rhys Anderson
Illustrator: Victoria Forest
References:
1. Kerepesi, C., et al., Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Science advances., 2021. 7(26): p. eabg6082.
2. Smits, M.A.J., et al., Human ovarian ageing is characterized by oxidative damage and mitochondrial dysfunction. 2023, Cold Spring Harbor Laboratory.
3. Gladyshev, V.N., The Ground Zero of Organismal Life and Aging. Trends in Molecular Medicine, 2021. 27(1): p. 11–19.
4. Zhang, B., et al., Epigenetic profiling and incidence of disrupted development point to gastrulation as aging ground zero in Xenopus laevis. 2022, Cold Spring Harbor Laboratory.
5. Clark, T.A., et al., Direct detection and sequencing of damaged DNA bases. Genome Integr, 2011. 2: p. 10.
6. Wang, F., et al., Solid-State Nanopore Analysis of Diverse DNA Base Modifications Using a Modular Enzymatic Labeling Process. Nano Lett, 2017. 17(11): p. 7110–7116.
7. Astigiano, S., et al., Fate of embryonal carcinoma cells injected into postimplantation mouse embryos. Differentiation., 2005. 73(9–10): p. 484–490.
8. Sahu, S., A. Dattani, and A.A. Aboobaker, Secrets from immortal worms: What can we learn about biological ageing from the planarian model system? Semin Cell Dev Biol, 2017. 70: p. 108–121.
9. Muller, W.A., Pattern formation in the immortal Hydra. Trends Genet, 1996. 12(3): p. 91–6.


