
The Science Behind NAD-Boosting & Anti-Aging

If you have been following the longevity field for even just a little a while, you have probably heard of NAD+… a lot. So what is NAD+, and why are people excited about it?

The Importance of NAD+ in Cellular Health
Nicotinamide adenine dinucleotide or NAD+ is essential for the fundamental biological processes of cellular health like energy production, metabolism, DNA repair, mitochondrial function, and more. NAD+ is vital for glycolysis, the citric acid cycle, and the electron transport chain, which is how the cells produce their energy. Moreover, NAD+ plays an important role in deacetylation, which is the removal of a specific molecular fragment called an acetyl group, driving numerous metabolic processes [1].
NAD+ is a Helper Molecule for Sirtuins and PARPs
NAD+ is a cofactor, or a “helper molecule,” for a large class of deacetylases called sirtuins [2]. These enzymes require a non-protein chemical cofactor like NAD+ to function. The effects of sirtuins go well beyond metabolism. They play key roles in inflammation, transcription of genes, programmed cell death, and — of course — aging. Different sirtuins are localized differently within the cell and have varying targets [3]: DNA modulation, energy homeostasis, and cell cycle control, just to name a few.
The sirtuins residing in the nucleus of the cell function as histone deacetylases and are vital for genome integrity and stability. Histones are proteins that DNA wraps around such that only the necessary bits are accessible and the rest is safely packed away. If there is a lack of NAD+, their function is reduced [4], the DNA loosens, and the histones are exposed to damaging external agents. Protecting genome integrity is essential for every cell to maintain its regular functions and characteristics.
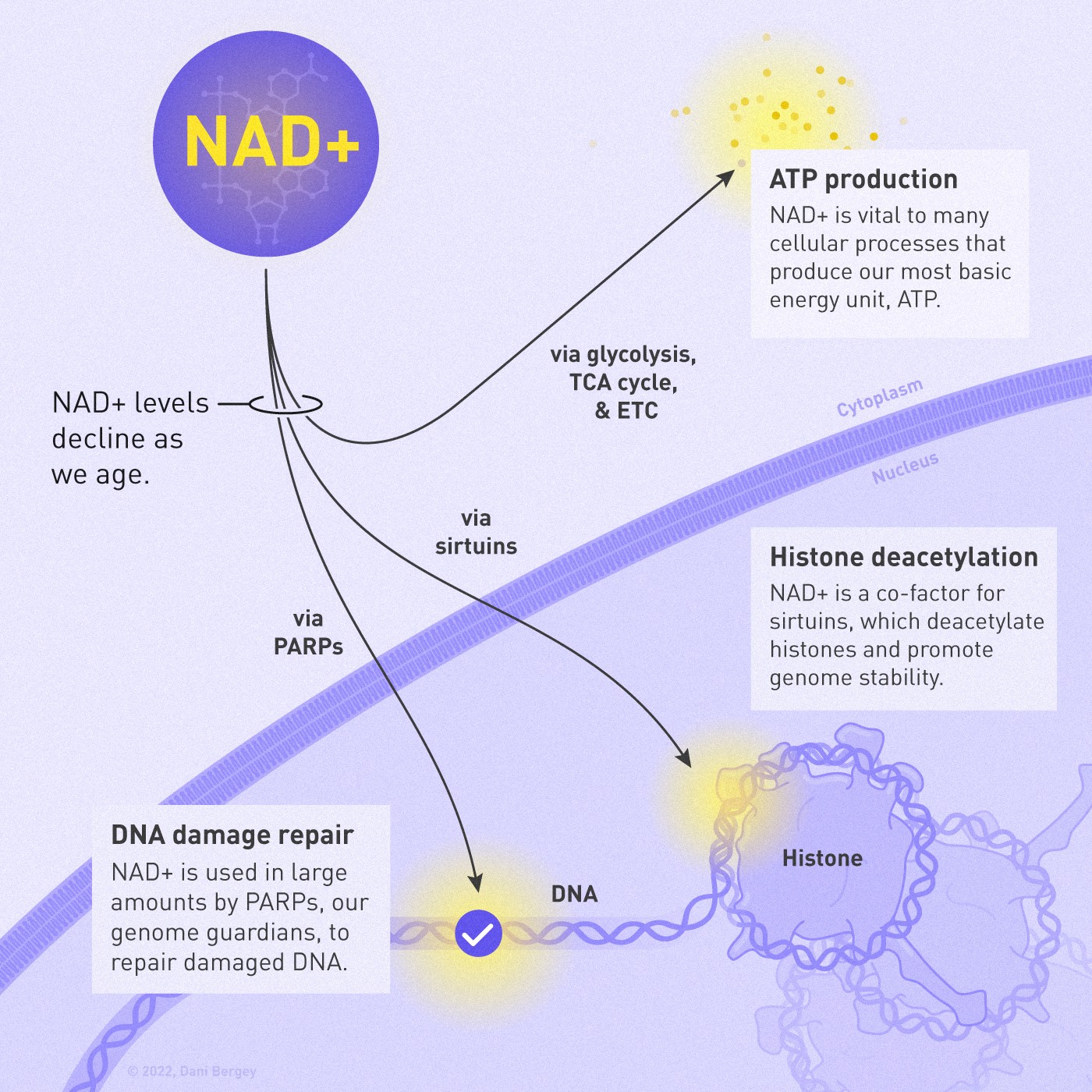
Another important family of proteins that require NAD+ are the DNA repairing proteins called PARPs [5] (poly (ADP-ribose) polymerase). DNA damage can accumulate in our cells as we age, and our cells try to do everything in their power to protect their DNA since it is the code book that regulates all life. More damage collected overtime would also require more repair, which is a problem in itself.
PARPs similar to sirtuins can also play a role in protein post translational modifications [6] via adding ADP-ribose units to certain proteins [7], a process called parylation. At the moment, much less is understood about how parylation impacts gene expression and protein function than acetylation/deacetylation, but it is expected that this part of the NAD+ field will grow rapidly in the coming years.
NAD+ Levels Decline with Age
Our genome guardians — the PARPs — use NAD+ in large amounts. As we age, there is a lot of DNA damage, which can start to exhaust the NAD+ supplies. This could be one reason why we see NAD+ decline as life progresses. Another reason is a well-known NAD+ consumer enzyme called CD38 [8]. We know that inhibiting CD38 [9] leads to an increase in available NAD+.
We find ourselves in this cellular Catch-22: there’s more DNA damage as we age, and we need NAD+ to repair it, but there is less NAD+ available with age, and we exhaust it even further when we repair DNA.
The availability and balance of NAD+ within the cell are crucial for health, and they are dependent on the rate of degradation and production of the molecule. Recent studies [10] show that NAD+ production is maintained with age but is used up more quickly as animals get older, leading to a 30% median decline in the tissues of 25-month-old mice (approximately equivalent to 80-year-old humans). While the mysteries behind this age-related decline are not yet completely understood, PARPs and CD38 are the main suspects.
This drop in NAD+ levels has proven to have detrimental consequences throughout the body [11] observed, across the brain, liver, muscles, vasculature, heart, kidney, immune and reproductive systems. A lot of the decline has been shown in studies to be reversible. We know we can fight the dropping levels by supplying the organism with NAD+ precursor molecules. This way we tip the scales in favor of NAD+ production and offset the increased degradation.
Potential Benefits of Boosting NAD+ Levels
Benefits from NAD+ boosting supplements may be seen across various systems in the body. Administration of NAD+ precursors has the potential to reverse neural degeneration [12], improve synaptic plasticity and memory [13] in Alzheimer disease animal models, and delay the senescence [14] of neural stem cells in old mice. In the liver, boosting NAD+ levels might be useful to prevent or treat diseases such as alcoholic and non-alcoholic steatohepatitis (NASH) [15], obesity [16], and more, as the evidence from animal studies suggest. In the muscles, human studies suggest it could be helpful against sarcopenia [17] (muscle wasting and weakness) as it appears to aid mitochondrial function, energy ATP production, inflammation reduction, and ultimately results in functional benefits such as increased endurance for running.
In the vascular systems [18] of multiple animal models, an increase of NAD+ levels seems to be effective at improving blood flow, restoring density of the capillary network and improving endothelial function for artery stiffness. In one large study on elderly mice, an NAD+ precursor supplementation showed a promising increase in blood flow, capillary density, and endurance [19]. In the heart [20] itself, boosting NAD+ has been shown to attenuate the progression of heart failure and specifically cardiomyopathy in mouse models.
A study suggests that NAD+ boosters could also act like anti-inflammatory agents, as they inhibit the expression of inflammatory markers and reduce chronic low-grade inflammation [21] (known as inflammaging) in mice. This decrease of inflammation, especially in certain tissues like the adipose tissue (fat), could mean a reduction in insulin resistance and obesity [22].
Lastly, beneficial effects are seen in the fastest aging system — the female reproductive system [23] — where NAD+ booster supplementation improves oocyte quality [24], ovulation rate, and as a functional outcome, the number of live births in aged mice.
Raising NAD+ Levels with Diet and Exercise
Some non-pharmacological ways to increase your NAD+ are diet and exercise [25]. Aerobic and resistance training for the duration of 12 weeks both were shown to ameliorate the age-induced NAD+ decline by increasing levels of the enzyme nicotinamide phosphoribosyltransferase (NAMPT.). These regimes also improved functional health measurements such as BMI, lean body mass, and glucose infusion rate.
NAD+ is synthesized via three different pathways from nicotinic acid, tryptophan, or precursors like NMN (nicotinamide mononucleotide) and NR (nicotinamide riboside). These molecules are bioavailable, and consuming a healthy balanced diet can provide a sufficient amount for a young person. Foods particularly rich in NAD+ precursors are milk, fish, yeast, green vegetables and whole grains. This, however, might be insufficient to battle age-related decline.
When compared to other bioavailable precursors like NA (nicotinic acid) and NAM (nicotinamide), NR is by far the most efficient. But how does it compare to NMN — the rising star in the NAD+ field?
Raising NAD+ Levels with Supplements: NMN vs NR
Both NMN and NR are available as supplements, and it is often debated which one is more effective. The current debate on whether one is better than the other is based on two main points: which one is more readily taken up by the cells and which one is more efficiently utilized.
One main difference is that NMN is a bigger molecule with an extra phosphate group, which means it is harder for NMN to enter the cell. NMN is the one, however, which directly produces NAD+. On the other hand, NR more easily enters the cell, but then it needs to be converted to NMN before it becomes NAD+.
Some studies [26] show that NMN needs to be converted to NR in order to cross the cell membrane, at which point the NR is converted back to NMN and subsequently to NAD+. Recently, a new NMN transporter [27] was found in the gut of mice, suggesting NMN can be directly transported into cells after all. According to genomic datasets, this transporter is also expressed in humans [28]. Its expression levels vary between different tissues, and closer study is required to determine what portion of NMN, if any, passes through that transporter in humans. The evidence that this transporter is actually trafficking NMN has been disputed [29].
Other studies propose that NMN has to become NR only to enter certain types of cells [30], while it is readily accepted by others. It has been seen to enter the mouse small intestine as is, even with the bulky phosphate group. We still do not know how this translates to humans.
While there are differences, there are also many similarities. As described above, there are numerous age-related health conditions in which NAD+ levels are reportedly low. In animal models, supplementing with NMN or NR seems alleviate these disease states [31]. Mostly, the end results are quite comparable in animals, though more studies are needed in humans. Both molecules and their derivatives are in clinical trials for several diseases, such as heart failure [32], sarcopenia (muscle wasting) [33], insulin resistance [34], and kidney disease, [35] to name a few, and so far have been found to be safe in humans [36].
Consumer Caution and Due Diligence
As with any other bioactive molecule, NMN and NR must be administered with caution and precision, as high levels might have potentially harmful effects. For example, an increased ratio of NMN to NAD+ was shown to activate a metabolic sensor in neurons associated with axon degeneration [37]. Another study showed that NMN enhances the inflammatory environment [38] in a mouse cancer model and that proinflammatory pathways can be induced by NAD+ metabolism.
Importantly, none of those negative effects have been observed yet in human clinical trials. Moreover, as described above, other studies have suggested the exact opposite: that NAD+ supplementation can fight neural degeneration and promote anti-inflammatory effects. Still, it is important that consumers be aware the science behind NAD+ supplementation is still actively under investigation.
Both NMN and NR are available for purchase as dietary supplements. It is important to note that regulations on supplements are very lax compared with pharmaceuticals, so consumers must exercise caution and due diligence when purchasing. For example, a recent report [39] showed that a number of NMN supplements sold on Amazon contained a concentration of NMN far below the one indicated on the label, and many had no detectable NMN whatsoever. This of course is not evidence against NMN itself, but rather evidence of unreliable retailers. It should be noted that this report was initiated by a competitor brand selling NR, and NR supplements were not tested. We will focus on considerations for supplement quality in an upcoming piece, since this is a hot topic in the community.
Final Thoughts
In closing, there is a wealth of data suggesting that healthspan can be extended by maintaining youthful NAD+ levels in the body. Studies have explored this over a number of preclinical models, and we are expecting data from human clinical trials very soon. We hope further studies will help us determine which supplement is the superior NAD+ booster and how it is best utilized in people.
DISCLAIMER: THIS ARTICLE DOES NOT PROVIDE MEDICAL ADVICE. No material in this article is intended to be a substitute for professional medical advice, diagnosis or treatment. The text, images and other material contained in this article are for informational purposes only.
Maria Marinova is a molecular biology graduate and a PhD candidate in reproductive medicine (OBGN). Her work focuses on ovarian damage caused by aging or chemotherapy and exploring the potential of NAD+ boosting molecules on protecting against or reversing these negative effects. Her interests extend beyond the ovary and towards extending women’s healthspan and lifespan by means of delaying menopause or preventing premature menopause. Some of her work on this topic has been published (co-author) in Cell Reports demonstrating how NAD+ repletion extends fertility in aged animals.
Dani Bergey, MS is a professional medical illustrator based in Seattle, WA, USA. Her focus is in the strategic storytelling and visualization of cell and molecular biology. She has an academic background in neurobiology, mathematics, medical illustration, and multimedia art; and she is a founding member of UltraRare, a web3 collective at the intersection of science and art.
Ariella Coler-Reilly is an MD PhD candidate at Washington University in St. Louis studying the genetics of aging. She moonlights as a science writer and illustrator, currently working as a managing editor for the VitaDAO blog. She is passionate about public education, diversity & inclusion, and the intersection of science & web3.
References
[1] Zhao et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010
[3] Yamamoto et al. Sirtuin Functions in Health and Disease. Molecular Endocrinology. 2007
[8] Wu and Zhang. CD38-expressing macrophages drive age-related NAD+ decline. Nature Metabolism
[11] Zapata-Perez et al. NAD+ homeostasis in human health and disease. EMBO Mol Med. 2021
[13] Lautrup at al. NAD+ in Brain Aging and Neurodegenerative Disorders. 2019. Cell Metab
[22] Berg and Scherer. Adipose tissue, inflammation, and cardiovascular disease. 2005. Circ Res
[23] Bertoldo et al. NAD+ repletion rescues female fertility during reproductive ageing. Cell Rep. 2020
[27] Grozio et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab. 2019
[28] SLC12A8 solute carrier family 12 member 8 [ Homo sapiens (human) ] Gene ID: 84561
[39] ChromaDex. Quantitative Analysis of Twenty-Two NMN Consumer Products. 2021


