The First Longevity Drugs might be already among us — Drug Repurposing to Fight Aging
We are getting quite good at extending lifespan, or at least we are getting quite good at extending the lifespan of laboratory animals as complex as mice.
If you follow the progress in anti-aging research, you will read about an arsenal of molecules, strategies and pathways that seem to make animals live longer (sometimes by several folds) and healthier. These results make us believe that increasing our years of healthy life is possible. Although, as nothing can be that easy, the benefits of these strategies seem to decrease with the complexity of the animal models. If we could show beyond reasonable doubt that any of these interventions works in humans, even by extending our healthy life by only a couple of years, that would be a huge milestone for the anti-aging field that would greatly accelerate progress.
Aging is not currently considered a disease by any regulatory agency, and therefore testing treatments to extend healthy lifespan is highly disincentivized.
There are several obstacles separating us from that first step, though. Aging is not currently considered a disease by any regulatory agency, and therefore testing treatments to extend healthy lifespan is highly disincentivized, since no drug can be approved on that basis. Additionally, drug development is difficult, with more than 90% of new molecules failing before they reach clinical trials due to high toxicity and/or efficacy. Things get a little bit easier when our molecule has reached human trials and failure rates drop significantly, being the lack of efficacy for the chosen disease the main reason for compounds to be dropped at this stage. As the drug gets closer to market, the trials get more and more expensive in order to answer the demands of regulatory agencies, and economic reasons start having more weight in the decision of dropping the development of new treatments.
This process already takes 10–12 years and costs between $2–3 billion for drugs with recognized indications… imagine the timescales and costs for drugs aimed to increase healthy lifespan. Any time that can be saved could have a huge impact and greatly reduce costs. For example, at the level of clinical trials we could find good surrogate biomarkers, such as the so-called epigenetic clocks, instead of waiting for years until participants die. But what would happen if some of the drugs that are used on a daily basis by doctors everywhere had the potential to extend lifespan and we only needed to tweak them a bit? This is the idea behind drug repurposing.
Starting the Race Half Way Closer to the Finish Line
Drug repurposing consists of using already existing drugs for other purposes than the one for what they were originally created, and it can be done with molecules that went all the way through their regulatory approval for a different use, or with those that were left in the way for different reasons. Since these molecules at least would have demonstrated to be decently safe and bioavailable, it is not difficult to see the convenience of repurposing when we consider the failure rates in early drug discovery that we have previously mentioned. If drugs that are currently in use are found to be repurposable, we start the race half way closer to the finish line and what is left is to demonstrate that our molecule is effective for its new use.
Of course this is no small feat, but it can shorten a 10–12 years process to a 3–8 years one, saving millions of dollars and what is more important, a comparable amount of lives.
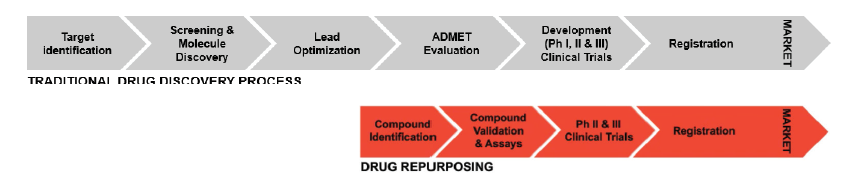
Figure from Kane, Anil. Whitepaper: Drug repurposing trends and strategic approaches for shortening timelines (https://patheon.com/resource-library/whitepapers/drug-repurposing-trends-and-strategic-approaches-for-shortening-timelines/)
If this is such a great strategy, why are we not seeing Big Pharma pursuing it more? Well, the pharmaceutical industry has been repurposing drugs successfully for years; sildenafil citrate (aka. Viagra) being probably the most well-known example. The blue pill was initially conceived as a treatment for angina pectoris, a heart condition, but interactions with important drugs in the management of this disease and the fact that it had to be taken three times a day made Pfizer drop that line of testing. Men participating in the clinical trials, however, experienced interesting side effects that convinced the pharmaceutical company to put the drug to a better use rather than abandoning it completely, resulting in the blockbuster drug for erectile dysfunction that we all know.
The issue with drug repurposing in the pharmaceutical industry is that it has been the result of serendipity, as the example of Viagra illustrates, rather than of a systematic approach.
AI and Decentralized Governance for Drug Repurposing
Given the potential benefits, there has been an increasing interest by the pharmaceutical industry on establishing repurposing programs, although the big players seem to encounter some challenges unlocking the full potential of drug repurposing and mainly focus on reusing their own old compounds. Some of these challenges include lack of good drug repositories and data, lack of collaboration within the industry and with academia, and maybe most importantly lack of financial incentive to find new uses for off-patent molecules.

That is why collaborative groups of academic researchers have taken over the task of repurposing generic drugs, lack of funding being their main limitation. All these pitfalls might explain why, despite the efforts, all the attempts to use already existing drugs to treat the SARS-CoV-2 infection have failed so far. One cannot avoid thinking if we would not have obtained better outcomes with more funding of independent researchers and transparent collaborations between companies in the industry.
Although traditional models of funding, IP and collaboration in the pharmaceutical industry do not seem to be particularly well-suited for this approach, a lot of exciting innovation has occurred in the repurposing space tackling both lack of data and the lack of incentives. Additionally, several companies have leveraged the power of AI to create interesting platforms for drug repurposing. Intellectual property sharing between stakeholders and crowdsourcing have also been proposed as possible solutions. At VitaDAO we are excited about the enabling potential that the IP-NFT framework has in this regard, in addition to the effects of decentralized governance on the creation of better incentives.
Metformin and Rapamycin: Old-style Repositioning Against Aging
But let’s bring the focus back to our mission: longevity. Drug repurposing is not an unkown approach in the anti-aging field: two of the most well studied compounds to extend lifespan in laboratory animals, metformin and rapamycin, were designed with very different aims.
Metformin has been used for decades to lower blood glucose in type 2 diabetic patients, being one of the most commonly prescribed drugs, and rapamycin is an immunosuppressant widely used to prevent organ rejection after transplants. Funnily enough, the current use of rapamycin is itself repurposing after the drug failed as an antifungal agent because it caused potent immune suppression as a side effect.
Metformin and rapamycin seem to work by acting on two different enzymes that function as energy sensors in cells: AMP-activated protein kinase and mammalian target of rapamycin (AMPK and mTOR for short) respectively. The activating effect of metformin on AMPK and the inhibitory effect of rapamycin on mTOR mimic some molecular aspects of calorie restriction and have been consistently shown to increase lifespan in model organisms.
Metformin and rapamycin are just the most well studied cases, but other molecules such as acarbose or the combination of growth hormone and dehydroepiandrosterone are in the candidate list.
Although large and comprehensive studies by the National Institute of Aging Intervention Testing Program cast doubt on the effectiveness of metformin to increase lifespan in mice, some claim that large clinical trials in non-diabetic patients are justified given the broad safety margin of the drug. Moreover, small trials in elderly patients have shown promising effect in markers associated with healthy aging. In fact, the Targeting Aging with Metformin (TAME) trial is currently raising funds in order to launch and hopes to make the treatment of aging an indication, which would be revolutionary in its own right.
Rapamycin, on the other hand, has a more narrow safety profile compared to metformin but has been consistently shown to extend lifespan in all animals where it has been investigated, and a clinical trial in humans has been recently crowdfunded to test its effect on several markers of aging, including epigenetic clocks.
Metformin and rapamycin are just the most well studied cases, but other molecules such as acarbose, used to inhibit the digestion of starch in diabetics, or the combination of growth hormone and dehydroepiandrosterone used for thymus regeneration are in the candidate list of compounds that could be repurposed to fight aging.
Can Serendipity be Systematized?
Besides these classical interventions, researchers are also trying to use more systematic approaches to find candidates for longevity drugs among our existing arsenal of compounds. A common way of doing this is to use databases of proteins or genes with a well-known role in aging and virtually screen libraries of compounds to predict which existing molecules will interact with the desired target.
Of course, these computational methods are just the first step in the repurposing path and need to be validated by experiments in model organisms, so one could argue that these approaches look similar to starting from scratch. That is why some researchers decide to skip the computational part and directly test a bunch of compounds in simple model organisms where you can get data on a quite large population and measure directly how they improve different parameters, including lifespan, with relative ease and without needing to care about the mechanism at first.
Some researchers decide to skip the computational part and directly test a bunch of compounds in simple model organisms as the worm Caenorhabditis elegans
A good example of this is the use of the worm Caenorhabditis elegans, which offers a convenient model of a small multicellular organism that shares some age-associated features with mammals. The problem with model organisms is that, obviously, they are not humans and sometimes drugs just do not work in the same way when you change species. This issue is called lack of translatability, and to tackle it some researchers try to directly use data from human patients and start from there to make predictions.
An interesting approach in this direction is to use existing data to look at how the expression of genes changes with aging in a certain tissue, and then look in existing databases for drugs that induce opposing changes, or similar changes if we suspect that they are adaptive, in cells. This has been successfully done for the human brain with a couple of promising molecules popping up after the analysis, including among them well known anti-aging drugs such as rapamycin. All these approaches have their advantages and disadvantages, and can certainly be combined to obtain more robust candidates, but all rely on the existence of available repositories with good quality data.
The Longevity Molecule: pushing for the anti-aging revolution

This takes us to the first project funded by VitaDAO: The Longevity Molecule by the Scheibye-Knudsen lab. Using exclusive access to the incredible wealth of real-world data contained in the medical and prescription records of the Danish Healthcare System, the research group led by Morten Scheibye-Knudsen has analyzed data from 4.8 million patients, including 1.04 billion prescriptions of 3500 different drugs over 40 years trying to find drugs that were correlated with a longer life.
Of course, people that get prescription drugs are not particularly healthy and how much they live depends more on their diagnosis than on the drugs they are taking. To avoid this “diagnosis bias” the Scheibye-Knudsen lab quantified the survival benefit of prescribed drugs compared to other drugs given for the same indication. Using this method, they found more than 10 medications that correlated strongly with lifespan in long-lived populations, and selected the three with a stronger correlation.
This project has the potential to uncover new mechanisms driving longevity
For the first part of the project, they will confirm the effects of these drugs in controlled laboratory experiments on human cells and on the well-known aging model Drosophila melanogaster (aka the fruit fly). If these experiments are successful, they will optimize them and begin testing on mice for the second phase of the project. Besides the goal of getting at least one candidate to start trials on human patients, this project has the potential to uncover new mechanisms driving longevity that could be targeted in the future.
If any of these approaches succeed, the next step under the current framework would be to choose an age-related disease to demonstrate an effect on human healthspan in the eyes of the regulatory agencies. Otherwise the framework should be changed to accept aging as a disease, but that is a different fight. We are still a long way from that, but only a few years of extra healthy life would be enough to change public perception and spark an anti-aging revolution. It is hopeful to think that the molecule able to do that could be already among us, hiding in plain sight.
Sources:
- Drug Repurposing Hub: https://www.nature.com/articles/nm.4306.epdf?author_access_token=lVts41U5Yg2SuJRRMDAHa9RgN0jAjWel9jnR3ZoTv0N3xDqwavSHuNFReme1zhqnnE8DcOdtv9bQ-OxQU2RQKy98QBCmKKsiYRvcMuCgpZl_tNkMVd1lNVJTAVS1n17M
- CTS article: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6226120/
- https://www.ddw-online.com/therapeutic-drug-repurposing-repositioning-and-rescue-part-iii-market-exclusivity-using-intellectual-property-and-regulatory-pathways-1239-201508/
- History of sildenafil: https://www.nature.com/articles/nrd2030
- New mechanisms to fund independent clinical research: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05273-x
- Repurposing Social Impact Bonds for Medicine: https://ssir.org/articles/entry/repurposing_social_impact_bonds_for_medicine#
- Pushpakom et al 2019. Drug repurposing: progress, challenges and recommendations (https://doi.org/10.1038/nrd.2018.168)
- PRNewswire press release: https://www.prnewswire.com/news-releases/molecule-partners-with-vitadao-and-nevermined-creating-first-ever-biopharma-ip-to-nft-transfer-for-longevity-research-301358287.html
- NIA interventions testing program (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5514387/)
- Aging medicine review metformin (https://onlinelibrary.wiley.com/doi/full/10.1002/agm2.12135#agm212135-bib-0082)
- TAME trial: https://www.afar.org/tame-trial
- PEARL trial: https://www.lifespan.io/campaigns/pearl-participatory-evaluation-of-aging-with-rapamycin-for-longevity/#description
- Identifying Potential Ageing-Modulating Drugs In Silico (https://www.cell.com/trends/endocrinology-metabolism/fulltext/S1043-2760(18)30211-X)
- Screening in C elegans: https://www.sciencedirect.com/science/article/pii/S0047637416301178?via%3Dihub
- Gene expression-based drug repurposing to target aging (https://onlinelibrary.wiley.com/doi/10.1111/acel.12819)


