EQUITY
A VITADAO PROJECT
Cyclarity - Novel Cyclodextrin Molecules for Multiple Aging-related Diseases
Funding
$50,000
Initiated
29.09.2023

Dr. Daniel M. Clemens
Vice President of Biology

Dr. Matthew O’Connor
Co-founder and Co-CEO

Michael Kope
Co-founder and Co-CEO
AT A GLANCE
Ongoing: Patent Status
PROJECT LINKS

Funding
$50,000
Initiated
29.09.2023

Dr. Daniel M. Clemens
Vice President of Biology

Dr. Matthew O’Connor
Co-founder and Co-CEO

Michael Kope
Co-founder and Co-CEO
AT A GLANCE
Ongoing: Patent Status
PROJECT LINKS
Cyclarity Therapeutics is an early stage biotechnology company developing computationally designed novel cyclodextrin drug molecules for the extraction of toxic biomolecules that accumulate with age and are implicated in a variety of age-related conditions.
Background
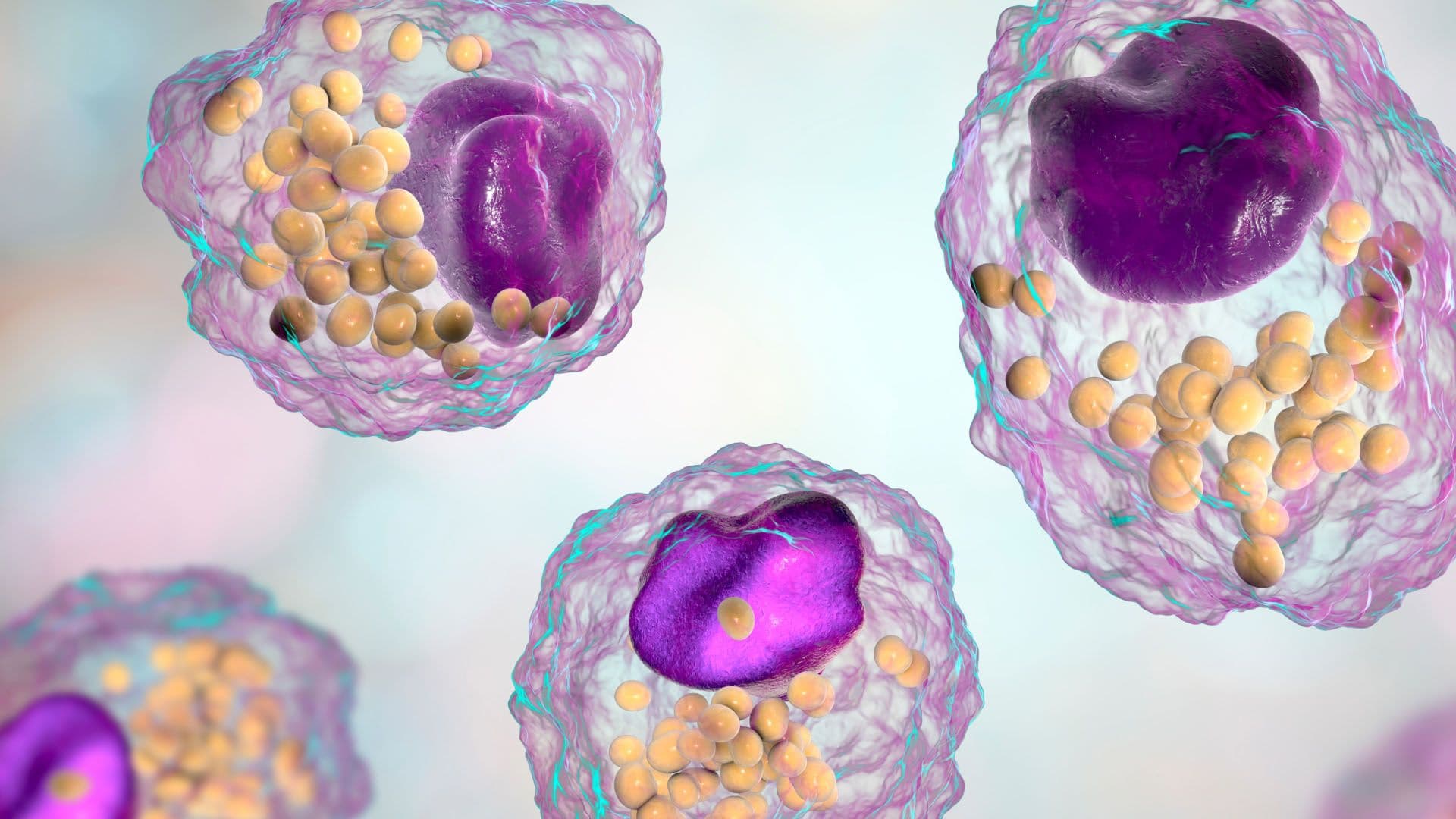
Aging is linked to the accumulation of several toxic biomolecules, notably 7-ketocholesterol (7KC), a form of oxidized cholesterol. This molecule naturally builds up in cells and has been pinpointed as a significant contributor to atherosclerosis, a disease that accounts for 44% of global deaths (based on 2017 data). Within atherosclerotic lesions, immune cells called macrophages initially work to clear the buildup. However, as they ingest 7KC mixed with other lipids, they become overwhelmed, turning into pro-inflammatory and dysfunctional foam cells, further aggravating the condition.
Aims, Hypothesis & Results
Cyclarity aims to combat toxic biomolecules associated with aging, primarily focusing on 7-ketocholesterol (7KC). Utilizing CDs (carbohydrates with a truncated conical shape) as active pharmaceutical ingredients (APIs), the goal is to bind these toxins effectively. Specifically, the development focus is on UDP-003, a novel class of CDs tailored for high affinity and specificity to 7KC.
The team hypothesized that UDP-003 would have a significantly higher affinity for 7KC than traditional monomeric CDs. By extracting 7KC from atherosclerotic vascular tissue and blood cells, it's expected that UDP-003 will reverse the toxic transformation of macrophages into foam cells, restoring their normal function and morphology.
UDP-003 demonstrated a remarkably higher affinity for 7KC compared to other CDs. It successfully extracted 7KC from human atherosclerotic tissues and transformed foam cells back to their original macrophage-like state. Additionally, pharmacokinetics revealed that UDP-003 had a short half-life and excellent bioavailability, especially through subcutaneous administration. Non-GLP safety-toxicology tests showed promising safety profiles, further solidifying its potential as a viable therapeutic option against atherosclerosis and other 7KC-related diseases.
Timeline
Cyclarity is currently raising funds for a $3-4M Bridge Round and have over $3M committed for this round. The bridge round funds will be used for final preparations and initiation of Phase 1 clinical trials. Additional funds will need to be raised to complete the Ph1 clinical trial.
Bridge Round:
Required Funding: $3.5M
Duration: Ongoing
VitaDAO Board Evaluation Writeup
Cyclarity Therapeutics is strategically positioned to address one of the most significant markets in aging-related diseases. Their innovative cyclodextrin (CD) molecules employ a unique mechanism to counteract toxic biomolecules that accumulate due to aging. The project boasts a robust intellectual property profile for this novel drug class and has displayed promising early results, outpacing competitors. The impending approach to clinical trials and the "pipeline in a pill" concept, where UDP-003 shows potential applicability across multiple conditions, further underscores its promise. Additionally, its platform's ability to target numerous age-related toxic biomolecules is commendable. However, the use of a new modality for cardiovascular disease poses risks, even if it has been successful in other domains. The project also faces stiff competition from emerging startups and potential resistance from established industry players. Furthermore, the intricacies of obtaining approval for cardiovascular drugs add to the challenges.
Latest Project Updates
4 May
2025
Cyclarity publishes promising data on atherosclerotic plaque reversal
Cyclarity published a paper on the Atherosclerosis journal suggesting that the targeted removal of 7-ketocholesterol (7KC) from foam cells with UDP-003 can potentially prevent and reverse atherosclerotic plaque formation. UDP-003, is their candidate drug that goes beyond managing atherosclerosis — it aims to reverse it by targeting one of its nastiest culprits, 7KC, a toxic oxidized form of cholesterol.
9 January
2025
Cyclarity enters clinical stage and raises series A
Cyclarity Therapeutics is pleased to announce regulatory approval to begin its first-in-human clinical trial. The trial will be conducted in Australia. In addition to a traditional SAD/MAD phase 1 trial, the authorization includes an allowance to enroll 12 patients with Acute Coronary Syndrome (ACS) to assess the safety of UDP-003 in individuals with plaque buildup, as well as to explore anecdotal evidence of efficacy.
In paralleled Cyclarity announces the closing of the first tranche of its Series A funding round, led by Ki Tua Fund LP and Starbloom Primrose LP. They will use funds from this round to commence clinical trials.
29 September
2023
Project Initiated